Blood ionic composition in marine elasmobranchs and holocephalans is some what higher than in teleosts, but its osmolarity is very much higher, close to that of seawater. This is because the blood contains, in addition to the usual ions, large amounts of low-molecular weight nitrogenous solutes, chiefly urea. First found in elasmobranchs in 1858, it is usually present at about 0.4 M. Various methylamine substances such as trimethylamine oxide (TMAO), betaine, and sarcosine, and some free amino-acids such as taurine and b-alanine are also present, in total around 0.2 M. So, over half of the osmolarity of the blood is due to these nitrogenous solutes, and, together with the inorganic ions, elasmobranch blood is osmotically close to seawater. Probably marine elasmobranchs are always slightly hyperosmotic to seawater, as for example at Plymouth, where dogfish (Scyliorhinus) in the aquarium circulation seawater then at 1154 mosmol kg–1, were found to have the serum at 1243 mosmol kg–1. The gills are permeable to water, so under normal conditions there will always be a slight influx of water excreted as urine that is more dilute than the serum or seawater.
Urea is a small molecule (MW: 60) and very soluble in water. How do elasmobranchs manage to retain it? Almost all urea loss is across the gills, some 20–70 mmol g–1 h–1 in those species examined, where a Na+-coupled urea transporter (UT) has been suggested in the gill, and where also, analysis of urea uptake revealed the presence of a phloretin-sensitive, Na+-coupled urea antiporter on the basolateral membrane of gill epithelial cells, which returns urea to the blood. In addition, the low lipid partition coefficient of urea means a slow rate of diffusion across the lipid bilayers of cell membranes, and the extraordinarily high cholesterol content in the basolateral membrane (cholesterol:phospholipid molar ratio 3.68) retards passive urea loss, as Fines et al. (2001), pointed out. Archer et al. (2004) have suggested a possible link between the elasmobranch array of neurohypophysial hormones, and urea-based osmoregulation.
The kidney must also conserve urea, and it does so with excellent efficiency. The most striking feature of the elasmobranch kidney is that 95% of the urea in the glomerular filtrate is resorbed. Passive urea absorption by facilitated diffusion into intracellular space (and thence to the blood) by a countercurrent system was first suggested by Boylan (1972) and the morphological basis for it was seen in the little skate Raja erinacea after long and painstaking reconstruction from digitized electron micrographs. The inner thinner part of the distal tubule is applied to the proximal tubule, making a series of lateral bundles in the kidney. The end result of this folding of the tubule back upon itself is that a countercurrent system of five parallel tubular segments is formed within a peritubular sheath, seemingly effectively resorbing urea and other nitrogenous solutes as the glomerular filtrate passes down the tubule to the collecting duct.
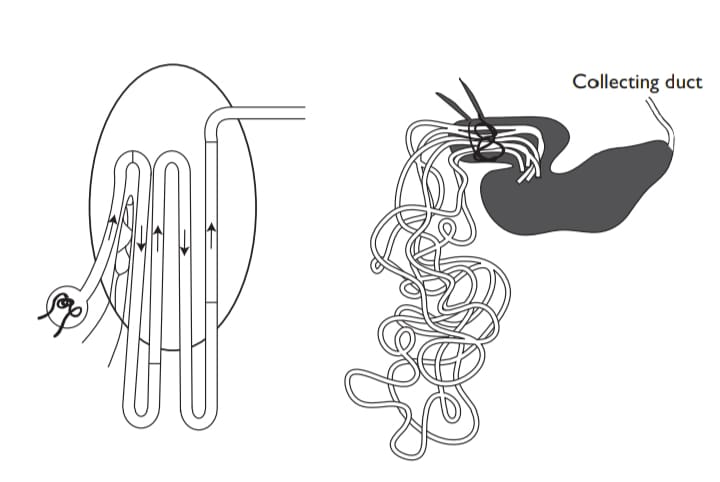
A most iconoclastic result was then more recently obtained in the kidney of the small shark Triakis scyllia, using the immunocytochemistry of antisera raised against a cloned cDNA for a specific UT (Hyodo et al., 2004). The UT was expressed exclusively in the final segment of the lateral bundle zone, that is in the collecting tubule of the kidney, suggesting that this was the main site for urea reabsorption. Na+/K+-ATPase was not detected in the collecting tubule, suggesting that transport (reabsorption) of urea in the collecting tubule occurs transcellularly by facilitated diffusion. The localization of UT on both apical and basolateral membranes supported this idea. Meanwhile, other nephron segments expressing Na+/K+-ATPase may function as an active component of urea transport.
So far, as Hyodo et al. (2004) were careful to point out, there has been no physiological evidence for urea permeability through the collecting tubule, and further results are needed before we can definitely conclude that the counter-current system of the elasmobranch kidney only plays at best a subsidiary role in urea resorption. The absence of a counter-current system in truly freshwater elasmobranchs (see below) is surprising if it does not play a role in urea resorption. In contrast to the limited localization of UT, the transport enzyme Na+/K+-ATPase is distributed in the basolateral membrane of numerous tubular segments both in the sinus zone and the bundle zone. However, in the collecting tubule, the role of the counter-current system may be to permit facilitated urea diffusion driven by Na+/K+-ATPase.
Tubular urea resorption is tightly coupled with sodium resorption at a fixed ratio of 1.6:1 over a wide range of urine flow rates and urea resorption values, indicating the presence of a sodium-coupled urea co-transporter. The hormones involved in the blood osmotic pressure regulation of elasmobranchs are still largely unknown. It has been suggested that the great diversity of oxytocin-like hormones in elasmobranchs expresses a release from an evolutionary receptor-binding constraint, so that amino-acid substitutions reflect neutral evolution. In contrast, the preservation of vasotocin suggests a selective pressure, which may be related to the regulation of renal urea transporter-recruitment mechanisms, as has been shown for vasopressin in mammals.
The oxidative fuel for elasmobranchs, unlike almost all other vertebrates, is provided not by lipids but by ketone bodies, such as b-hydroxybutyrate. Until recently, this unusual metabolism seemed likely to be linked to urea-based osmoregulation. However, Speers-Roesch et al. (2006) have examined a fresh water potamotrygonid ray, the euryhaline stingray Taeniura lymma, and a marine shark (each having different amounts of urea in the blood and other tissues, and find that there is no correlation between urea levels and ketone bodies. As T. H. Huxley once wrote, “The great tragedy of Science is the slaying of a beautiful hypothesis by an ugly fact!” When urea is not needed as an osmolyte, as in the freshwater rays, it is deaminated rather than shunted to urea production.


