According to Karel Liem (1980) most of the enormous variety and range of foods eaten by fishes is obtained through only three basic feeding styles: ram feeding, suction feeding, and manipulation or biting. Virtually all species use one, and, because they are not mutually exclusive, most species use two of these styles.
Much of the evolutionary change that has occurred in lineages leading to both modern sharks and bony fishes has involved the development of mouths and jaws that are more efficient in food gathering. Primitive Actinopterygians (Paleoniscoids) as well as living polypterids provide examples of jaw structures that are adapted to engulfing their food whole. The relatively inflexible jaws of these older lineages were limited to more-or-less opening and closing and contrast sharply with the protrusible jaws of modern teleosts that actually reach out to engulf their prey (Lauder, 1980a, b). Jaw protrusion is believed to have evolved independently at least five times: in sharks, in chondrosteans, in ostariophysans, and again in higher teleosts (chiefly acanthopterygians, but also in cods (Gadidae) perhaps uniquely among the paracanthopterygians). A key factor in the evolution of protrusible jaws in the acanthopterygian perciform lineage has been the increased importance of a highly mobile premaxillary and commensurate restriction of the maxillary as the primary structure in the upper jaw (Ferry- Graham and Lauder, 2001).
These fishes open their mouths by utilizing the hypaxial/sternohyoideus muscle to lower the mandible, but can also use their epaxial muscles to hinge the neurocranium upwards relative to the vertebral axis and thus increase the size of their gape. In its simplest form, expansion of the buccal cavity is very limited in these cases and a small, but adequate, negative pressure is developed; however these fishes may enhance their feeding efficiency by ram feeding using body velocity to overtake and capture prey.
Although ram and suction feeding may be viewed as extremes of a spectrum of feeding modes (Norton and Brainerd, 1993), many fish use a combination of forward motion of the body or jaw in addition to generating suction pressure to entrain prey. Barracuda (Sphyraena barracuda), pike (Esox), and gars (Lepisosteus) typically rely entirely on ram feeding, engulfing their prey by making a rapid lunge with their jaws wide open (Porter and Motta, 2004). The long-jawed butterflyfish (Forcipiger longirostris) rapidly extends its jaws while enlarging its gill chamber and reaching out, often into confined spaces where suction feeding alone would not be as effective. (Ferry-Graham et al., 2001).
Ram feeding occurs when a fish swims with its mouth open through a concentration of food. The mouth may be held open continuously (or for very long periods) or intermittently. Food may then be sieved from the water by gill rakers, collected in sticky mucus, or otherwise routed into the esophagus where itis then swallowed. Ram feeding is sometimes referred to as “passive feeding,” however, the fish must actively expend energy for swimming and a streamlined fish swimming with its mouth open loses much of its hydrodynamic efficiency thus increasing the cost of swimming and making ram feeding much less energy efficient than it might at first appear to be. This hydrodynamic loss may be partially compensated for by the injection of water from the gill openings into the boundary layer similar to what happens during respiration.
Studies on herring and other species have shown that a fish may use ram feeding at high concentrations of plankton, but switch to “picking” individual prey (suction feeding) at lower concentrations. At moderate concentrations, when prey would be equally available by either means, fish often favor picking over ram feeding, suggesting that suction feeding is energetically more efficient (Gibson and Ezzi, 1992). In contrast to ram feeding, most fishes use suction to ingest their prey. The buccal and opercular chambers expand, drawing water, and any prey organisms in that water, into the fish’s mouth.
Suction feeding is used to capture many different types of foods, for example: suspension feeding on dense aggregates of microorganisms or detritus, plankton picking, crevice feeding, bottom vacuuming, and by sit-and-wait predators. Suction feeding typically consists of four phases: (1) preparatory – a decrease in volume of the mouth by compression of the head and elevation of the hyoid; (2) expansive – which occurs progressively from front to rear, beginning with the opening of the mouth and subsequent enlargement of the gape, depression of the hyoid, abduction of the suspensorium, and abduction of the operculum (during which the opercular opening remains closed to prevent the escape of water); (3) compressive – closing of the jaws, adduction of the suspensorium and protraction of the hyoid, while opening the opercular flap; resistance of the gill arches delaying the outflow of water and permitting retention of food; and finally (4) recovery – a return to conditions preparatory for phase 1 (Horn, 1998).
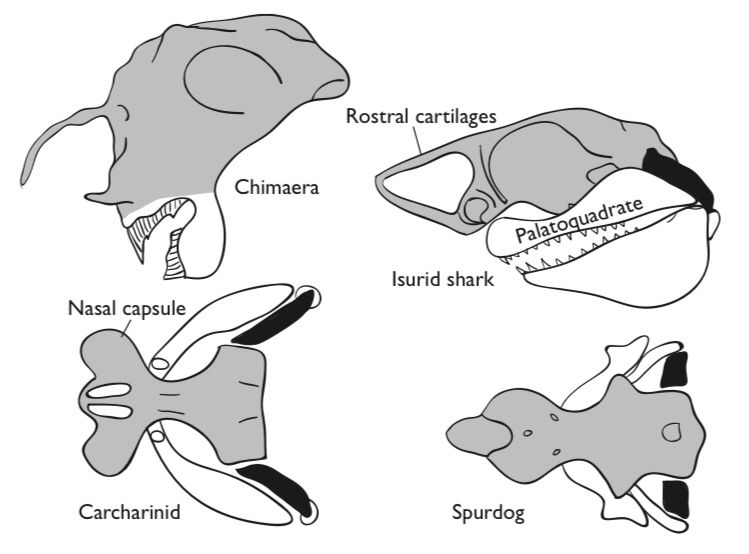
Suction feeding occurs in a wide variety of fishes and has been suggested as the primitive feeding style for all bony fishes (Hulsey et al., 2005). The absence of teeth associated with the earliest fossils from the Lower Silurian (420 mya) suggests to some that these were also filter feeders. Functional convergence in suction feeding between living sharks and bony fishes reflect similar hydrodynamic constraints resulting from evolutionary pressures to develop greater feeding efficiencies through protrusible jaws in both lineages. Relationships between jaw suspension and feeding in sharks are not, however, as clear as formerly believed (Wilga, 2002). Upper jaw protrusion in elasmobranchs is related to morphology of the upper jaw-chondrocranium articulation rather than to the type of jaw suspension as had been previously suggested.
Interestingly, the same jaw morphology and musculature that permits suction feeding can also be adapted to allow the fish to eject a stream of water that can be used to uncover buried prey or manipulate prey so it can be more easily consumed. Dasyatid and myliobatid stingrays forage by jetting water out of their ventrally oriented gill openings to blow large pits in the bottom (Wilga et al., 2007), and these have been recognized in fossil sediments. The ability of archer fish (Toxotes) to “shoot down” insects with jets of water has already been mentioned and triggerfishes (Balistidae), which feed on echinoderms, use a jet of water to uncover buried sand dollars or overturn long-spined sea urchins, allowing them to attack the undefended ventral side.
Biting, as defined by morphologists and biomechanicists, involves the use of the jaws to grasp prey and includes feeding on only parts of a larger prey organism, taking bites of flesh, or of fins, scales, eyeballs, parasite picking, or benthic scraping found in many species of coral reef fishes and rock-dwelling cichlids in which the jaws are applied to the substrate or directly to the prey. When defined this way, biting becomes the most specialized mode of feeding, and is believed to have evolved several times: in sharks (and perhaps earlier in placoderms), in cichlids, plectognath fishes (triggerfishes and their relatives), and in algal scrapers in diverse families including loaches, catfishes, blennies, and cichlids.
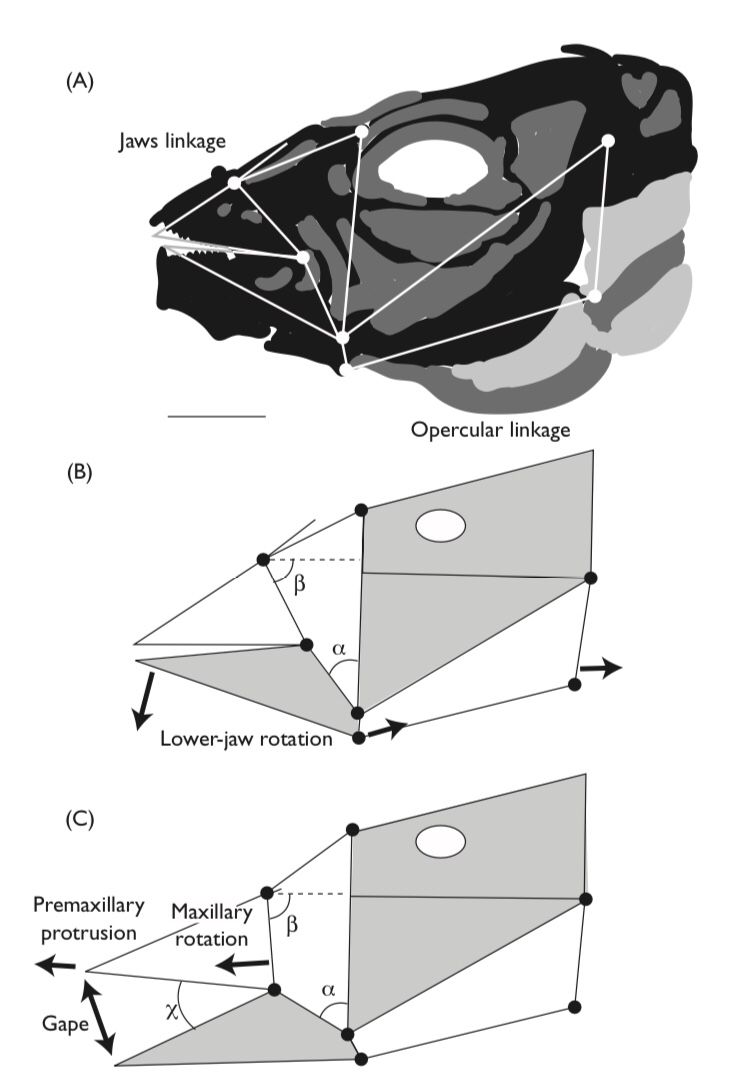
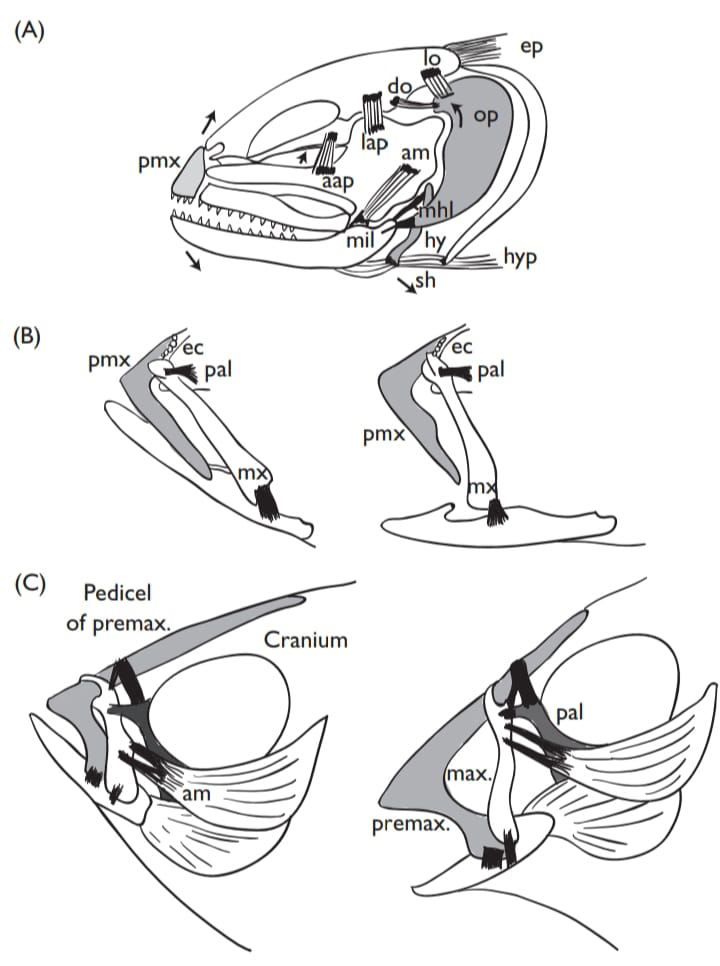
Regardless of the mode of feeding the feeding apparatus of most fish consists of four-bar-linkage systems constructed from the bones of the skull that form an expandable mouth cavity shaped like a truncated cone. In contrast to simple lever systems, such as the lower jaw, most of which possess an input and an output link that rotate around a fulcrum provided by the jaw joint, four-bar linkages have a third link that transfers the transmission of motion and force as well as a fourth, stationary, link that anchors the assembly. Four-bar linkages are found not only in the mouth but also the pharyngeal and opercular apparati and these multiple linkages work together to permit the capture and processing of food.
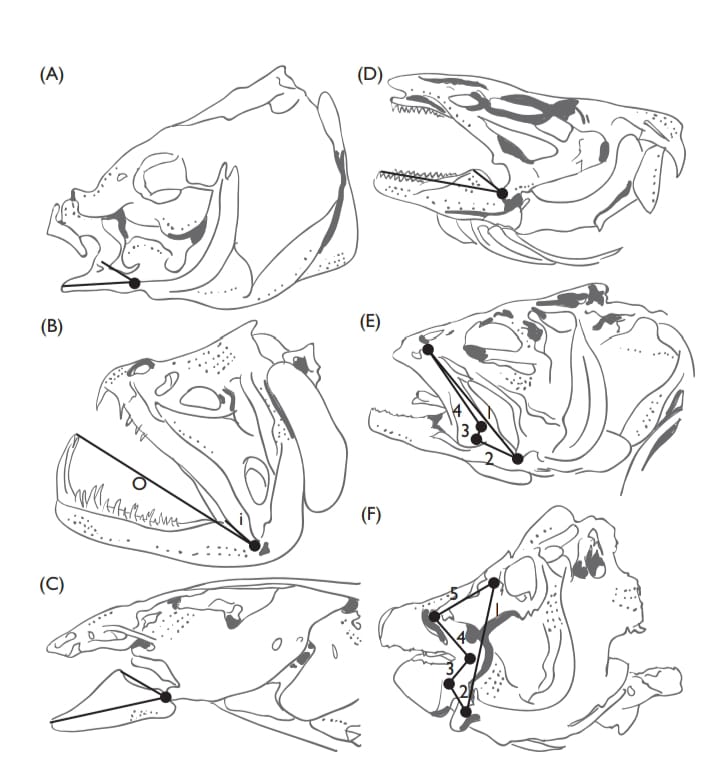
Expansion of the mouth cavity produces negative (suction) pressure that assists in the ingestion of food. Naturally, the actual feeding mechanism of fishes is more complex than this simple model and differences in the relative sizes and position of each bony element in the linkage system as well as associated muscles and tendons can produce a great variety of results, ranging from very rapid and forceful to very slow and precise feeding movements (Higham et al., 2006;). Most suction feeders are intermittent, however, lampreys and their ammocoete larvae possess anatomical structures that permit more-or-less continuous suction feeding currents.


