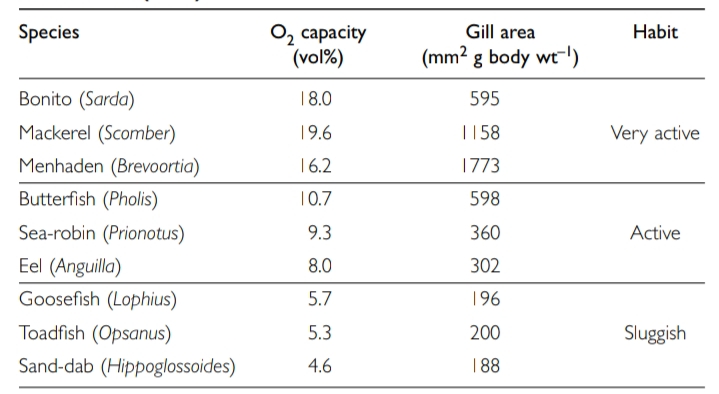
Different fishes have very different lifestyles, so it is not surprising that the properties of their blood vary according to metabolic demands, and the way that the fish acquires O2 and excretes CO2. For example, blood in active fishes such as scombroids must have a much higher O2 capacity than in sluggish fishes such as angler fish; in obligate air-breathers it must be less sensitive to CO2 content than it is in water-breathing fishes. Note that the O2 capacity of whole blood comprises O2 in solution in the blood plus O2 combined with hemoglobin; this red cell respiratory pigment raising the O2-capacity up to 40 times.
However, high oxygen capacity with a high hematocrit incurs costs in increased blood viscosity, (Sadler et al., 2000) and these are not trivial since O2-carrying capacity increases linearly with hematocrit, while the linked increase in viscosity is exponential. Fish can reduce blood viscosity when stressed, since the adrenergic stress response releases circulating catecholamines which make red cells swollen, but also more deformable presumably by acting on their surface receptors.
Remarkably, in several Antarctic icefishes in the family Chaenichthyidae, blood hemoglobin is much reduced or totally lacking, and there are no red blood cells. All the O2 reaching the tissues must do so in solution in the blood, which has the same O2 carrying capacity as seawater, viz. around 0.7 vol%, compared with around 8 vol% in many normal fishes with hemoglobin. To overcome the low O2-capacity of the blood, icefishes have relatively large gills, well-vascularized skin for cutaneous respiration, large hearts with large cardiac output and large-diameter blood vessels; to reduce O2 demand they have reduced their red aerobic myotomal musculature.
Unexpectedly, as O’Brien et al. (2002) have shown, the large red myotomal fibers have almost double the density of mitochondria seen in the fibers of notothenioids with hemoglobin. Since this cannot mean enhanced aerobic capacity, the suggestion is that the mitochondria aid in intracellular oxygen transport as O2 is more soluble in lipid than in aqueous cytoplasm. The resting O2 uptake of icefishes is from one-half to two-thirds that of fishes in the same habitat that possess hemoglobin, and they survive by a combination of such adaptations, low metabolic rate, high cardiac output, and living at low temperatures (when blood O2 capacity is high). It remains a mystery why they should have lost hemoglobin.
The early larvae of many teleosts also lack hemoglobin (although, of course, it appears later in development), and so do the long-lived leptocephali larvae of eels, where it is reasonable to guess that it is lacking to complete their glassy transparency. But even in fish such as trout, pike (Esox) and goldfish, O2 dissolved in the blood suffices for resting metabolism. When these fishes are poisoned with CO (so that the hemoglobin cannot combine with O2), they survive well until exercise increases O2 demand, when they perish.


