A cilium or flagellum has considerable internal structure. Each flagellum or cilium contains nine pairs of longitudinal microtubules arranged in a circle around a central pair and this is true for all motile flagella and cilia in the animal kingdom, with a few notable exceptions. This “9+ 2” tube of microtubules in a flagellum or cilium is its axoneme; an axoneme is covered by a membrane continuous with the cell membrane covering the rest of the organism. At about the point where an axoneme enters the cell proper, the central pair of microtubules ends at a small plate within the circle of nine pairs. Also at about that point, another microtubule joins each of the nine pairs, so that these form a short tube extending from the base of the flagellum into the cell. The tube consists of nine triplets of microtubules and is known as a kinetosome (or basal body). Kinetosomes are exactly the same in structure as centrioles that organize mitotic spindles during cell division.
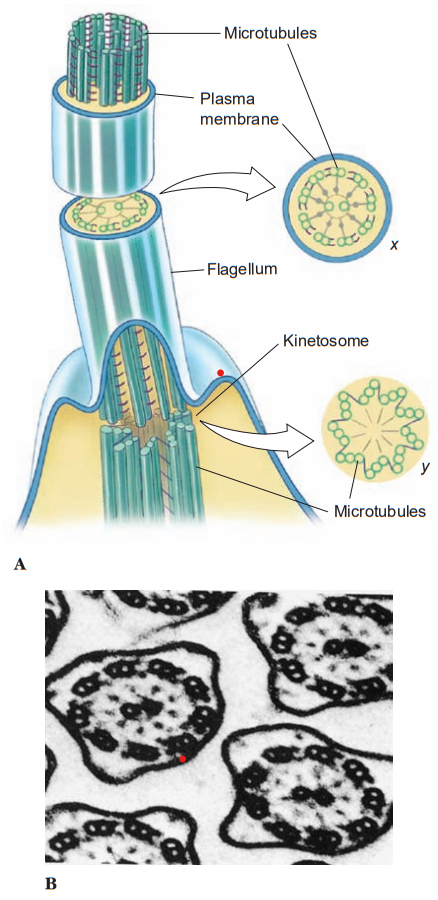
Centrioles of some flagellates may give rise to kinetosomes, or kinetosomes may function as centrioles. All typical flagella and cilia have a kinetosome at their base, regardless of whether they are borne by a protozoan or metazoan cell. Many small metazoans use cilia not only for locomotion but also to create water currents for their feeding and respiration. Ciliary movement is vital to many species in such functions as handling food, reproduction, excretion, and osmoregulation.
The current explanation for ciliary and flagellar movement is the sliding microtubule hypothesis. The movement is powered by a release of chemical bond energy in ATP. Two little arms composed of the protein, dynein, are visible in electron micrographs on each of the pairs of peripheral tubules in the axoneme, and these bear the enzyme adenosine triphosphatase (ATPase), which cleaves the ATP. When bond energy in ATP is released, the arms “walk along” one of the filaments in the adjacent pair, causing it to slide relative to the other filament in the pair.
Shear resistance, causing the axoneme to bend when the filaments slide past each other, is provided by “spokes” from each doublet to the central pair of fibrils. These spokes are visible in electron micrographs. Direct evidence for the sliding microtubule hypothesis was obtained by attaching tiny gold beads to axonemal microtubules and observing their movement microscopically.
Pseudopodia
Pseudopodia are extensions of the cell cytoplasm used in locomotion. The cytoplasm is not homogeneous; sometimes peripheral and central areas of cytoplasm can be distinguished as ectoplasm and endoplasm. Endoplasm appears more granular and contains the nucleus and cytoplasmic organelles. Ectoplasm appears more transparent by light microscopy, and it bears the bases of the cilia or flagella. Ectoplasm is often more rigid and is in the gel state of a colloid, whereas the more fluid endoplasm is in the sol state.
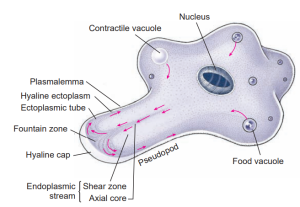
Ameba in active locomotion. Arrows indicate the direction of streaming endoplasm. The first sign of a new pseudopodium is thickening of the ectoplasm to form a clear hyaline cap, into which the fluid endoplasm flows. As the endoplasm reaches the forward tip, it fountains out and is converted into ectoplasm, forming a stiff outer tube that lengthens as the forward flow continues. Posteriorly the ectoplasm is converted into fluid endoplasm, replenishing the flow. Substratum is necessary for ameboid movement.
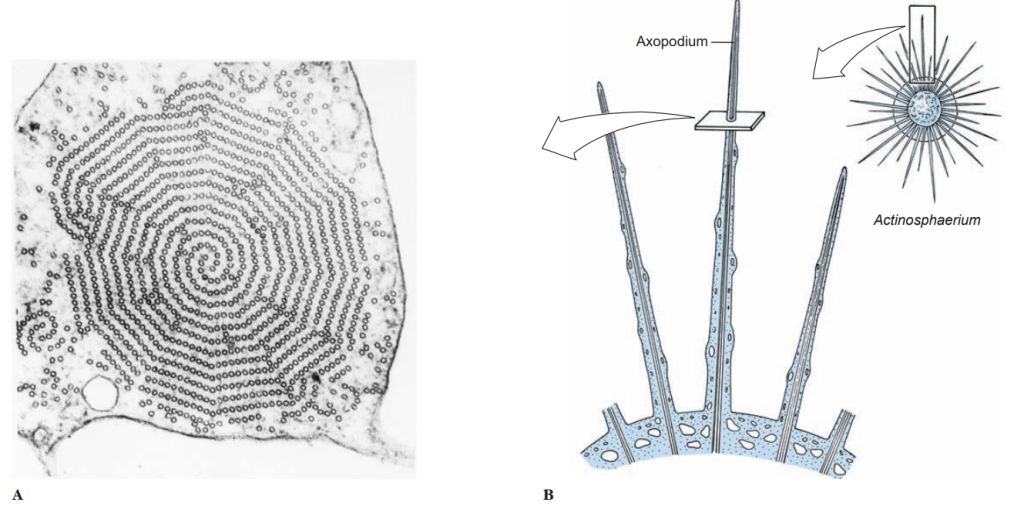
Pseudopodia vary in composition and are of several types. The most familiar are lobopodia, which are rather large, blunt extensions of the cell body containing both endoplasm and ectoplasm. Some amebas characteristically do not extend individual pseudopodia, but movem the whole body with pseudopodial motion; this movement is known as the limax form (for a genus of slugs, Limax). Filopodia are thin extensions, usually branching, and containing only ectoplasm. They occur in some amebas, such as Euglypha. Reticulopodia are distinguished from filopodia in that reticulopodia repeatedly rejoin to form a netlike mesh, although some protozoologists consider the distinction between filopodia and reticulopodia artificial. Members of superclass Actinopoda have axopodia, which are long, thin pseudopodia supported by axial rods of microtubules.
The microtubules are arranged in a definite spiral or geometrical array, depending on the species, and constitute the axoneme of the axopod. Axopodia can be extended or retracted, apparently by addition or removal of microtubular material. Since the tips can adhere to the substrate, the organism can progress by a rolling motion, shortening the axonemes in front and extending those in the rear. Cytoplasm can flow along the axonemes, toward the body on one side and in the reverse direction on the other.
Although pseudopodia are the chief means of locomotion in amebas, they can be formed by a variety of flagellate protozoa, as well as by ameboid cells of many animals. In fact, much defense against disease in the human body depends on ameboid white blood cells, and ameboid cells in many other animals, vertebrate and invertebrate, play similar roles.
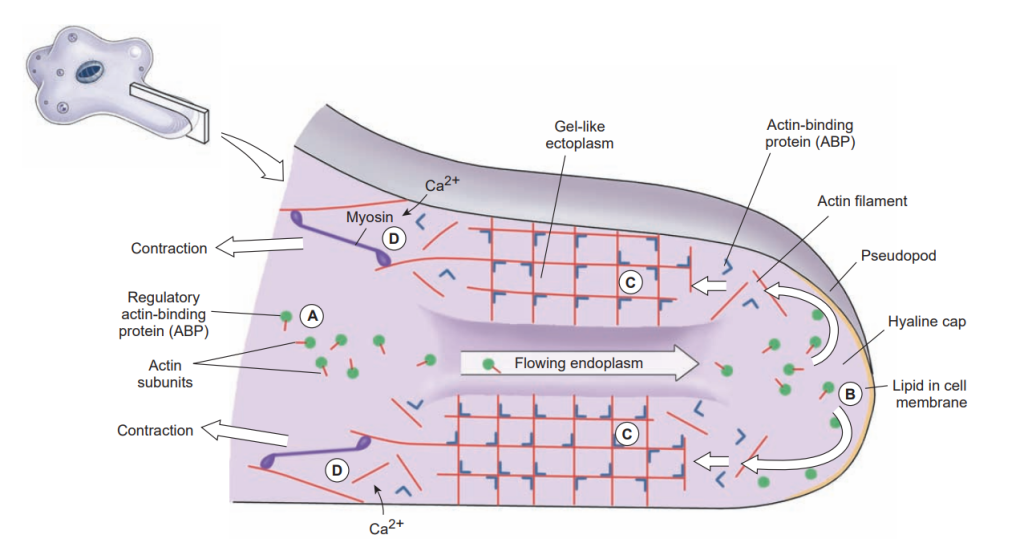
When a typical lobopodium begins to form, an extension of ectoplasm called a hyaline cap appears, and endoplasm begins to flow toward and into the hyaline cap. The flowing endoplasm contains actin subunits attached to regulatory, actin-binding proteins (ABPs) that prevent actin from polymerizing. As endoplasm flows into the hyaline cap, it spreads to the periphery. Interaction with phospholipids in the cell membrane releases the actin subunits from their regulatory binding proteins and allows them to polymerize into actin filaments.
The actin filaments become crosslinked to each other by another ABP to form a semisolid gel, transforming the ectoplasm into a tube through which the fluid endoplasm flows as the pseudopodium extends. Near the trailing edge of the gel, calcium ions activate an ABP that releases actin filaments from the gel and permits myosin to associate with and to pull these actin filaments. Thus contraction at the trailing edge creates a pressure that forces the fluid endoplasm, along with its now-dissociated actin subunits, back toward the hyaline cap.
Useful External Links
- protozoan: Media by Britanica
- What is locomotion in protozoa? by Vedantu
- Classification of Intestinal Protozoa by Locomotion by LabCE