The Oparin-Haldane hypothesis stimulated experimental work to test the hypothesis that organic compounds characteristic of life could be formed from the simpler molecules present in the prebiotic environment. In 1953, Stanley Miller and Harold Urey in Chicago successfully simulated the conditions thought to prevail on the primitive earth. Miller built an apparatus designed to circulate a mixture of methane, hydrogen, ammonia, and water past an electric spark. Water in the flask was boiled to produce steam that helped to circulate the gases. The products formed in the electrical discharge were condensed in the condenser and collected in the U- tube and small flask (representing an ocean).
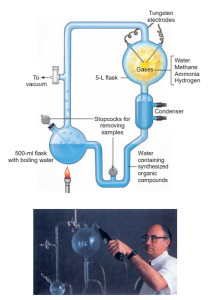
After a week of continuous sparking, approximately 15% of the carbon from the reducing “atmosphere” had been converted into organic compounds that collected in the “ocean.” The most striking finding was conditions not greatly different from those that Miller simulated.
In other experiments, electrical discharges were passed through mixtures of carbon monoxide, nitrogen, and water, yielding amino acids and nitrogenous bases. Although reaction rates were much slower than in atmospheres containing methane and ammonia, and yields were poor in comparison, these experiments support the hypothesis that the chemical beginnings of life can occur in atmospheres that are only mildly reducing. The need for methane and ammonia, however, led to proposals that these substances might have been introduced by comets or meteorites or that they were synthesized near the hydrothermal vents.
Thus the experiments of many scientists have shown that highly reactive intermediate molecules such as hydrogen cyanide, formaldehyde, and cyanoacetylene are formed when a reducing mixture of gases is subjected to a violent energy source. These molecules react with water and ammonia or nitrogen to form more complex organic molecules, including amino acids, fatty acids, urea, aldehydes, sugars, and nitrogenous bases (purines and pyrimidines), all of the building blocks required for the synthesis of the most complex organic compounds of living matter. Further evidence for the natural abiotic synthesis of amino acids comes from finding amino acids in meteorites, such as the Murchison meteorite that landed in Australia in 1969.
Formation of Polymers
The next stage in chemical evolution involved the joining of amino acids, nitrogenous bases, and sugars to yield larger molecules, such as proteins and nucleic acids. Such synthesis does not occur easily in dilute solutions, because excess water drives reactions toward decomposition (hydrolysis). Although the primitive ocean might have been called a “primordial soup,” it was probably a rather dilute one containing organic material that was approximately one-tenth to one-third as concentrated as chicken bouillon.
Need for Concentration
Prebiotic synthesis must have occurred in restricted areas where concentrations of the reactants were high. Violent weather on the primitive earth would have created enormous dust storms; impacts of meteorites would have lofted great amounts of dust into the atmosphere. The dust particles could have become foci of water droplets. Salt concentration in the particles could have been high and provided a concentrated medium for chemical reactions. Alternatively, perhaps the surface of the earth was too warm to have oceans but not too hot for a damp surface. This condition would have resulted from constant rain and rapid evaporation. Thus, the earth’s surface could have become coated with organic molecules, an “incredible scum.” Pre bioticm molecules might have been concentrated by adsorption on the surface of clay and other minerals. Clay can concentrate and condense large amounts of organic molecules. The surface of iron pyrite also has been suggested as a site for the evolution of biochemical pathways. The positively charged surface of pyrite would attract a variety of negative ions, which would bind to its surface. Furthermore, pyrite is abundant around hydrothermal vents, compatible with the hydrothermal vent hypothesis.
Thermal Condensations
Most biological polymerizations are condensation (dehydration) reactions, in which monomers are linked together by the removal of water. In living systems, condensation reactions always occur in an aqueous (cellular) environment containing appropriate enzymes. Without enzymes and energy supplied by ATP, macromolecules (proteins and nucleic acids) of living systems soon decompose into their constituent monomers.
Dehydration reactions could have occurred without enzymes in primitive earth conditions by thermal condensation. The simplest dehydration is accomplished by driving water from solids by direct heating. For example, heating a mixture of all 20 amino acids to 180° C produces a good yield of polypeptides.
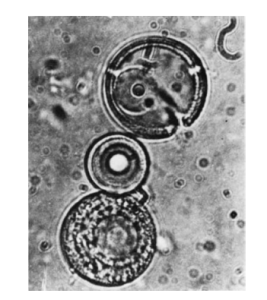
The thermal synthesis of polypeptides to form “proteinoids” has been studied extensively by the American scientist Sidney Fox. He showed that heating dry mixtures of amino acids and then mixing the resulting polymers with water forms small spherical bodies. These proteinoid microspheres possess certain characteristics of living systems. Each is not more than 2 µm in diameter, comparable in size and shape to spherical bacteria. The outer walls of the microspheres appear to have a double layer, and they show osmotic properties and selective diffusion. They may grow by accretion or proliferate by budding like bacteria. Proteinoids might have been used to assemble the first cells from macromolecular precursors. Formation of these polymers requires conditions likely to have occurred only in volcanoes. Organic polymers might have condensed on or in volcanoes and then, wetted by rain or dew, reacted further in solution to form polypeptides or polynucleotides.
External Useful Links
- Oparin-Haldane Hypothesis by StudySmarter
- What is Oparin Haldane theory? Can life be originated abiotically inside the laboratory today? by Vedantu
- Oparin-Haldane theory by Britanica
 EazyBio: Educate, Elevate, Empower EazyBio: Educate, Elevate, Empower
EazyBio: Educate, Elevate, Empower EazyBio: Educate, Elevate, Empower




